Ubiquinol Water-dispersive Powder (ShiroQ)
Evolution of Coenzyme Q10
- No antioxidant effects.
- Must be converted to Ubiquinol CoQ10 in the body.
- The ability to convert decreases with age and stress.
- Absorption is low unless taken with oil because it is fat-soluble.
- An antioxidant effects.
- Works directly in the body without any conversion.
- Absorption is low unless taken with oil because it isfat-soluble.
That's why we developed “Water- soluble emulsion technology”.
More energy can be produced.
- Absorption is about 3 times higher than before
- Possible to process tablets in any shape
- Works quickly and efficiently in the body
-Characteristics-
- Processed into water-dispersive powder using Water-soluble emulsion technology.
- Absorption is approximately three times higher than soft capsules in the body.
- Works quickly and efficiently on the body.
- Choose from a wide variety of final products, including drinks, hard capsules, jellies, tablets and etc..

Powder

Water-dispersive
-Specifications-
| Items to test | Specification | Test method |
|---|---|---|
| Appearance | off white - fine yellow | Visual inspection |
| Content of Ubiquinol | NLT 40.0% | HPLC method |
| Heavy metal | NMT 10μg/g | Food Sanitation Law(JP) |
| Number of live bacteria | NMT 300cfu/g |
Standard Methods of Analysis in
Food Safety Regulations(JP)
|
| Total Coliforms | Negative |
Standard Methods of Analysis in
Food Safety Regulations(JP)
|
-Research-
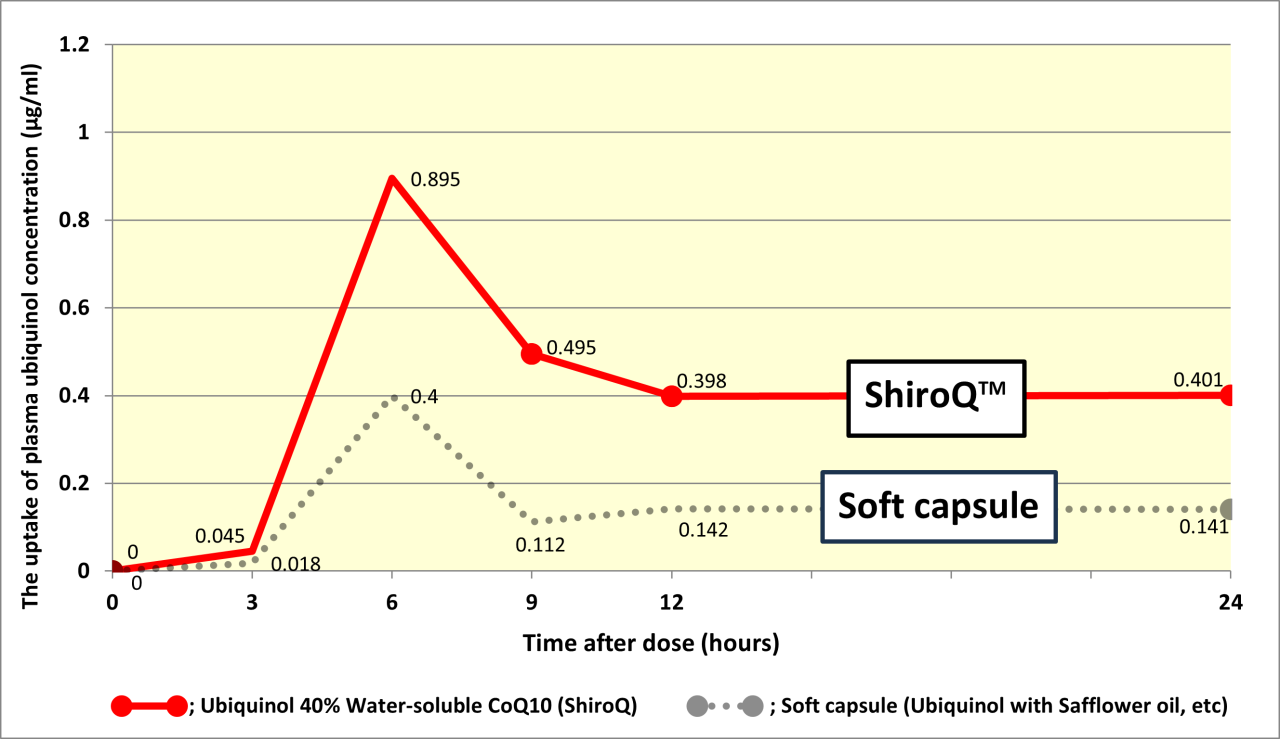
In this study, we showed the bioavailability of ubiquinol (QH) in the form of Ubiquinol 40% water-soluble CoQ10(ShiroQ). Two groups of 5 healthy young subjects received single oral administration of 100 mg of QH in the form of a soft capsule containing QH dissolved in safflower oil or Ubiquinol 40% water-soluble CoQ10(ShiroQ) in the fasting period, and changes in the plasma QH concentration were monitored over time. The plasma QH concentration before administration was 0.68 ± 0.08 μg/ml. Tmax was 6 hours after the administration. Cmax values compared with the pre-administration baseline in the soft capsule and Ubiquinol 40% water-soluble CoQ10(ShiroQ) groups were 0.4±0.21 and 0.89±0.27 μg/ml, respectively, and AUC0-24hr values were 3.59±1.63 and 9.68±2.35 μgh/ml, respectively. Ubiquinol water-soluble CoQ10(ShiroQ) of QH exhibited superior bioavailability even when administered in the fasting period.
Japanese Journal of Complementary and Alternative Medicine Vol.11,No2, September,2014
Author; Yoshihiro UCHIDA1, Kouichi WAKIMOTO1, Hidehiro TAKAHASHI2, Kenji FUJII3
1Seisen orthopedic clinic,2Petroeuroasia Co.,Ltd.,3KANEKA CORPORATION
-Solution comparison-

Kaneka bulk powder

ShiroQ

Soft capsule





 Contact us
Contact us