Ubiquinol Water-dispersive Powder-E (ShiroQE)
-Characteristics-
- Processed into water-dispersive powder using water-soluble emulsion technology.
- Absorption is approximately three times higher than soft capsules in the body.
- Works quickly and efficiently on the body.
- Choose from a wide variety of final products, including hard capsules, jellies, tablets and etc..
- Non-hygroscopic type.


-Specifications-
| Items to test | Specification | Test method |
|---|---|---|
| Appearance | off white - fine yellow | Visual inspection |
| Content of Ubiquinol | NLT 30.0% | HPLC method |
| Heavy metal | NMT 10μg/g | Food Sanitation Law(JP) |
| Number of live bacteria | NMT 300cfu/g | Standard Methods of Analysis in Food Safety Regulations(JP) |
| Total Coliforms | Negative | Standard Methods of Analysis in Food Safety Regulations(JP) |
-Research-
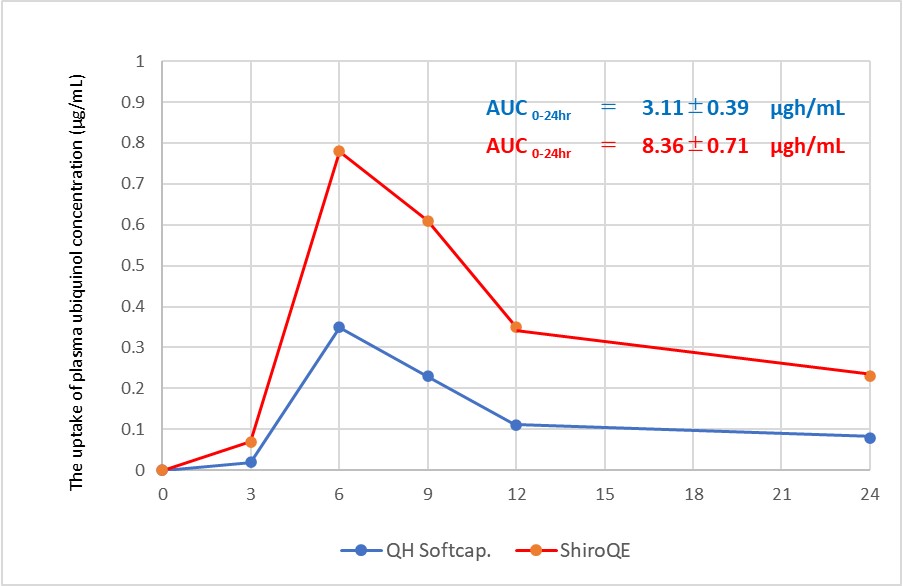
In this study, we showed the bioavailability of Ubiquinol 30% Water-dispersive Powder-E (ShiroQE). 4 healthy subjects received single oral administration of 100mg of QH in the form of a soft capsule※ containing QH dissolved in Safflower oil and a ShiroQE in a hard capsule in the fasting period, and changes in the plasma ubiquinol concentaration were monitored over time. AUC0-24hr values were 3.11 and 8.36μgh/ml, respectively. 4 healthy subjects did not take any coenzyme supplements or drinks for 3 weeks, and did not take anything but water from 6:00 p.m. on the day before to 6:00 p.m. on the day of the trial. The same 4 subjects repeated the trial in the same manner.
※ingredients of commercial soft capsule:Ubiquinol, Safflower oil, glycerin, glycerin fatty acid ester, lecithin, vitamin E, etc.
-Patent-
US. 11911350.B2
EP.3760196
JP.7273278


 Contact us
Contact us